Research Overview
Giardia lamblia, the causative agent of giardiasis, leads to gastrointestinal disorders, long-term growth defects and even death. Estimates place worldwide incidence at over 200 million symptomatic cases per year. Of concern, up to 20% of cases are resistant to front-line treatments, and resistance to all major anti-giardial drugs has been reported. In addition to their increasing lack of effectiveness, front-line and second-line nitro drugs act intracellularly via non-specific free radical generation, and therefore have a high incidence of negative side effects including disruption of beneficial intestinal microflora. There is a critical need to better understand the basic biology of the parasite in order to ultimately design improved treatment strategies.
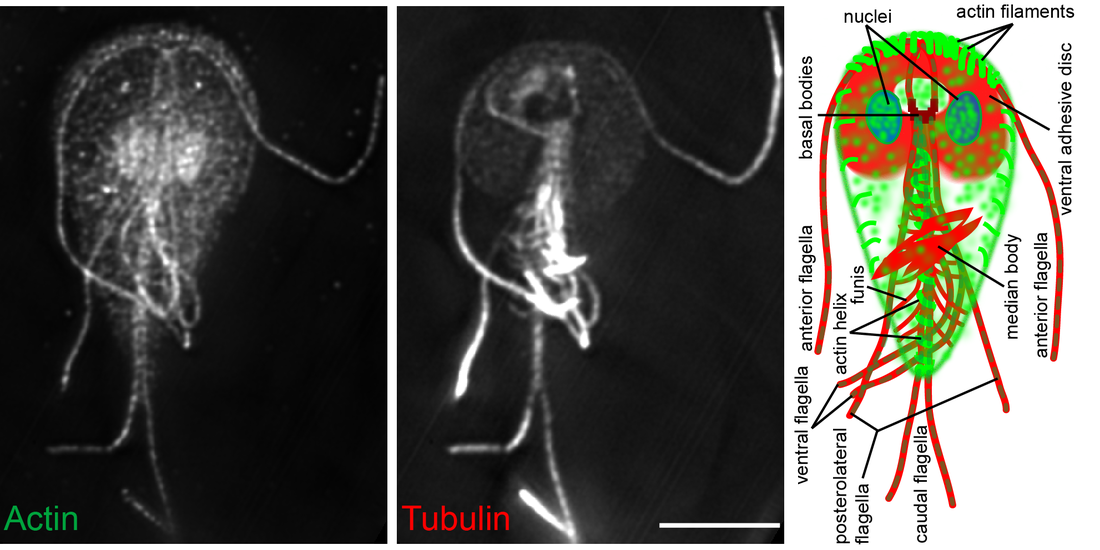
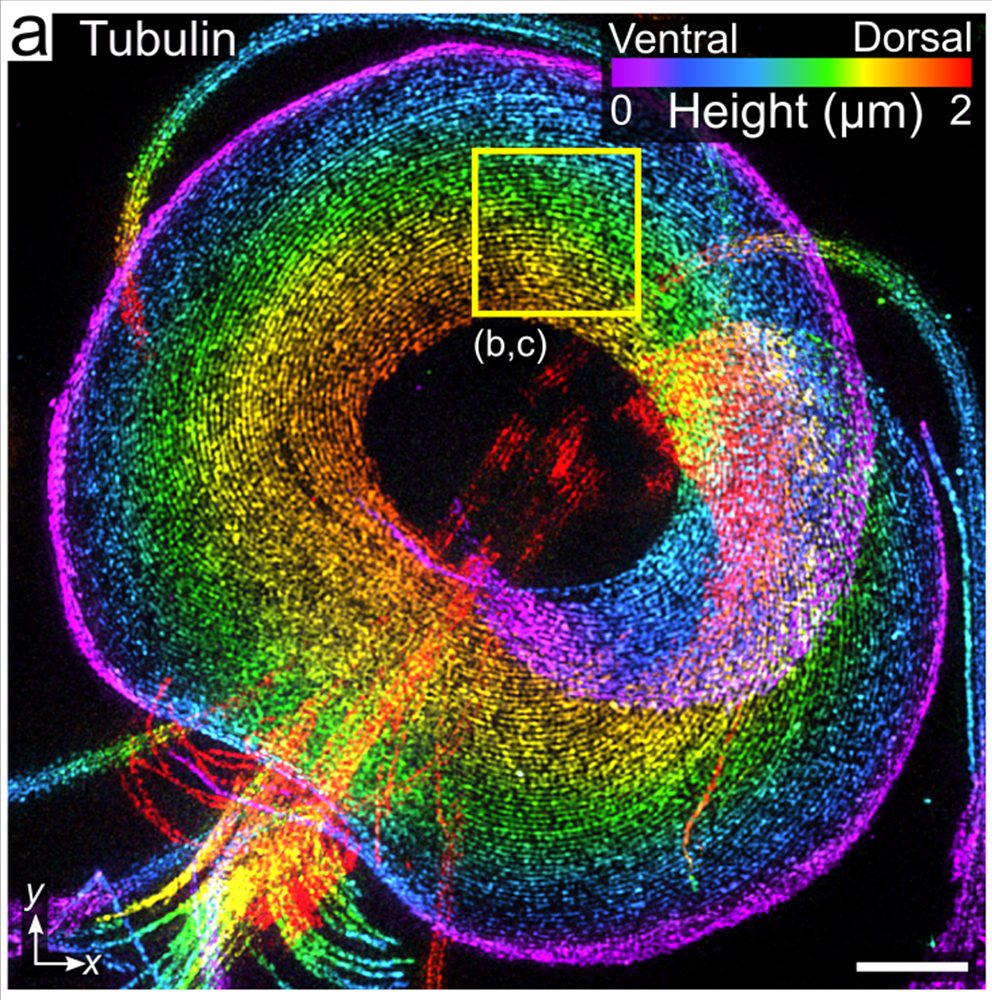
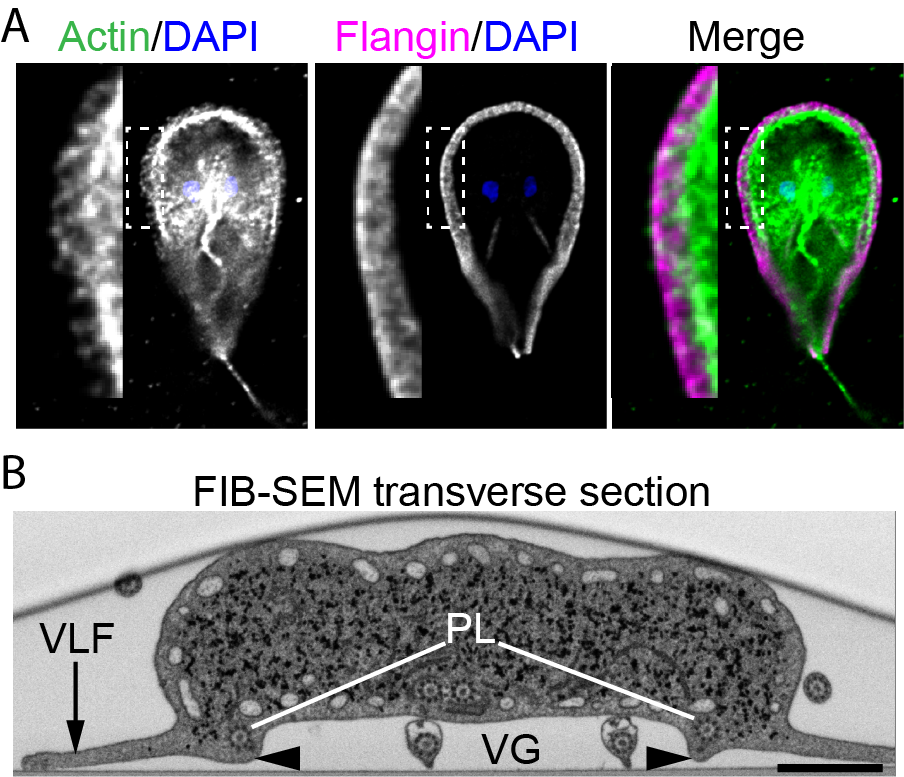
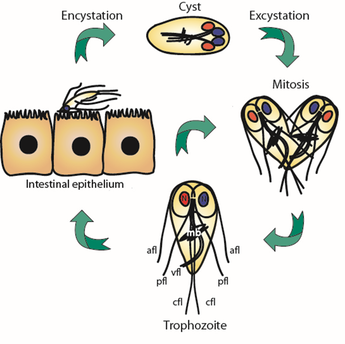
Flagellated trophozoites proliferate to colonize the intestine where they use their cytoskeleton to build adhesive structures that support attachment to the host intestine. Cues including cell density, low cholesterol levels, and increased pH at the end of the intestinal tract promote terminal differentiation into cysts that results in a halt to proliferative growth and disassembly of the cytoskeletal structures that support attachment. While current treatments target Giardia’s anaerobic metabolism, we envision targeting the signaling pathways that regulate the cytoskeleton and the encystation process to clear infections. Due to the highly divergent nature of Giardia’s actin cytoskeleton the mechanisms regulating its function in parasitic attachment are poorly understood, yet the highly divergent yet essential actin cytoskeleton is a likely drug target. The regulation of encystation across the diversity of encysting parasites is poorly understood; thus, studies focused on the encystation pathway are of fundamental cell and developmental biology interest, as well as profound clinical relevance. We aim to identify the means to short circuit the normal encystation program such that cell cycle arrest and cytoskeletal disassembly can be activated without producing a protective cyst wall. This would clear infections without the risk of transmission.
Flagellated trophozoites proliferate to colonize the intestine where they use their cytoskeleton to build adhesive structures that support attachment to the host intestine. Cues including cell density, low cholesterol levels, and increased pH at the end of the intestinal tract promote terminal differentiation into cysts that results in a halt to proliferative growth and disassembly of the cytoskeletal structures that support attachment. While current treatments target Giardia’s anaerobic metabolism, we envision targeting the signaling pathways that regulate the cytoskeleton and the encystation process to clear infections. Due to the highly divergent nature of Giardia’s actin cytoskeleton the mechanisms regulating its function in parasitic attachment are poorly understood, yet the highly divergent yet essential actin cytoskeleton is a likely drug target. The regulation of encystation across the diversity of encysting parasites is poorly understood; thus, studies focused on the encystation pathway are of fundamental cell and developmental biology interest, as well as profound clinical relevance. We aim to identify the means to short circuit the normal encystation program such that cell cycle arrest and cytoskeletal disassembly can be activated without producing a protective cyst wall. This would clear infections without the risk of transmission.
Current Projects:
|
Signaling Regulating the Cytoskeleton and Encystation
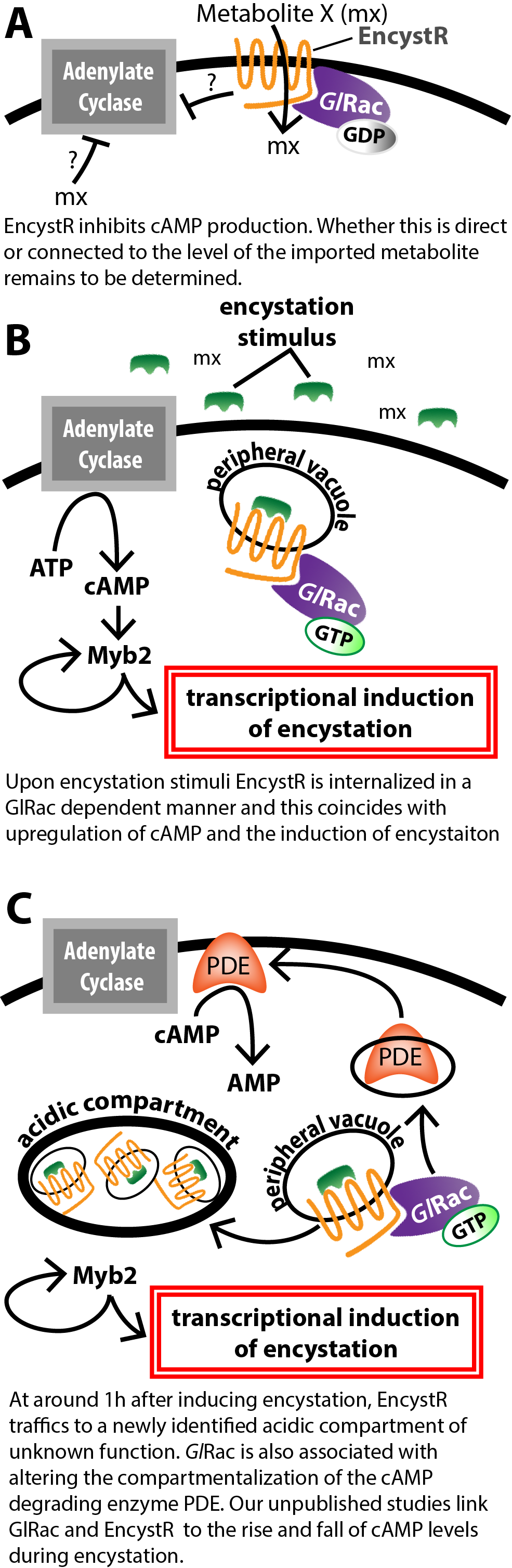
Upstream of ABPs in plants and animals are Rho family GTPases which act as local signaling platforms that have an important role in coordinating cytoskeleton and membrane trafficking proteins. We have shown that Giardia’s sole Rho GTPase, GlRac has a conserved role in regulating the cytoskeleton and membrane trafficking [5, 8-10]. We also found that GlRac has a role in regulating encystation. To find GlRac effector proteins we performed a protein-protein interaction study that that led to the identification of EncystR. EncystR is a plasma membrane localized transmembrane protein that functions as a negative regulator of encystation. Notably, EncystR represents a novel control point in Giardia’s developmental program. Since the encystation response involves a halt to proliferative growth and major alterations to cell shape and the cytoskeleton that result in parasite detachment, we envision that hyperactivation of encystation signaling is an excellent strategy to clear infection. Moreover, protozoan members of every eukaryotic supergroup form resting cysts, but how this is regulated remains poorly understood. Giardia is arguably the most developed parasite model for studying encystation, positioning us at the forefront of this area of single cell development. Our work on EncystR is still underway (see working model on right). Preliminary studies have determined that EncystR is linked to cAMP signaling, which regulates development in many protozoans. Additionally preliminary studies indicate that EncystR has a role in nutrient transport (we are not ready to share the identity of this nutrient), indicating that EncystR is a transporter-receptor (transceptor). We are now working to connect the entire pathway from signal perception to the activation of the transcriptional program regulating encystation. Starting at transcription we recently developed a novel Giardia knockdown system and used this to systematically test the role of all early upregulated transcription factors in Giardia [11]. We identified novel regulators of encystation that function within the first 30 minutes of inducing encystation. Now that we have identified which transcription factors are key to regulating entry into the encystation pathway we are set to uncover the role of cAMP in their activation. We will then move upstream to work on how EncystR connects to cAMP signaling. Ultimately, our goal is to identify means to short circuit the normal encystation program such that we can activate cytoskeletal disassembly without forming a functional cyst wall, which is necessary to protect the parasite outside of the host. Therefore, short circuiting the normal pathway would induce detachment from the intestine, but the parasites would not be able to survive outside of the host for transmission. EncystR and the associated downstream signaling network is an ongoing area of research in the laboratory. |